

Fireworks at an atomic level:
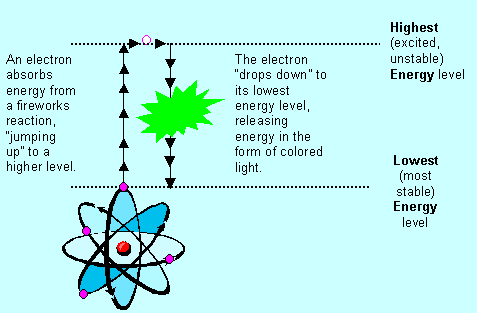
Electrons always want to be in the closest possible energy level to the nucleus. If the atom absorbs any form of energy, the electron is forced into a higher energy level. Since the new energy level is unstable, the electron returns back to the original energy level. The only way for the electron to return to the ground state is if the electron releases the energy. The release of the energy results in a release of a photon of light. This also determines the color of the light. The point where the photon of light is emitted is referred to as the flash point. The visible light spectrum ranges from red to purple. The blue and purple end of the spectrum is a higher energy compared to the red end. If an atom picks up a lot of energy, it will release a color towards the purple end of the spectrum and vice versa.
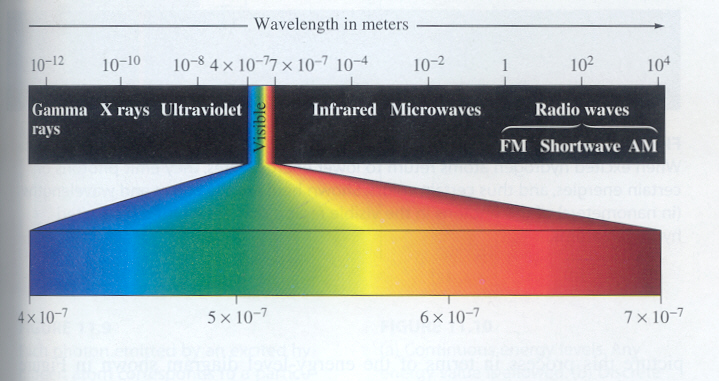
Wavelength:
The colors involved with fireworks are contain on the visible light spectrum. As the wavelength increases the amount of heat needed to create the reaction decreases. The purple end of the spectrum requires the most heat and has the shortest wavelength. The red end of the spectrum has the longest wavelength on the visible spectrum.
Chemicals:
Different chemicals require different amounts of heat to create an energy
transfer. As stated before, the compounds that produce a red color require less
heat because it has a shorter wavelength. The electrons become excited at lower
energy levels and are able to produce a photon of light at a lower temperature.
| Color | Compound | Wavelength (nm) | |
| red |
strontium salts, lithium salts lithium carbonate, Li2CO3 = red strontium carbonate, SrCO3 = bright red
|
652 | |
|
|
orange |
calcium salts calcium chloride, CaCl2 |
668 |
|
|
yellow |
sodium salts sodium chloride, NaCl |
610-621 |
|
|
green |
barium compounds + chlorine
producer barium chloride, BaCl2 |
589 |
|
|
blue |
copper compounds + chlorine
producer copper(I) chloride, CuCl |
505-535 |
|
|
purple |
mixture of strontium (red) and copper (blue) compounds |
420-460 |
|
|
silver | burning aluminum, titanium, or magnesium |
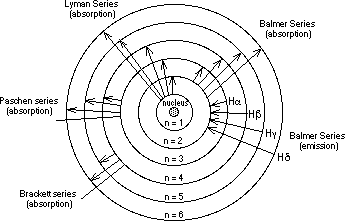
Blamer Series:
The arrangement of light separated according to wavelength, frequency, or
energy. The Balmer Series deals with the transitions starting or ending
with the first excited state of an atom.
Firework Anatomy
There are 7 main parts to the construction of an aerial firework.
Lift charge: The area towards the bottom
of the fireworks cylinder where heat and gases are trapped. The pressure from
teh heat and gas build up and eventually send the firework soaring into the sky.
Main Fuse: A fuse that is triggered by a computer that lights two other
secondary fuses within the firework. There is a fast-acting fuse and another
that is on time delay in the center of the firework. The fast acting fuse lights
the lift charge.
Launch tube: The launch tube is three times the length of the fireworks,
but always the same diameter. The firework must fit in tight into the tube so a
misfire does not occur. The launch tube is also referred to as a mortar.
Black powder: The powder that is ignited to start the explosion out of
the launch tube. Black powder is a simple formula involving potassium
nitrate, charcoal, and sulfur. Firework manufactures can manipulate the rate of
explosion by either using fine or coarse powder. The coarse powder burns at a
slower rate than fine powder.
Stars: Starts create the colors seen by the spectators. They are composed
of chemicals that are found on the table above. Each manufacture makes their own
color based on the types of chemicals used.
Time delay fuse: As the firework travels through the air, the time delay
fuse is still burning. When the firework has almost reached it highest point,
the fuse lights the black powder stored in the compartment.
Break: The compartments where the stars are stored. The time delay fuse
is also contained in this compartment. The compartment is made with very heavy
wrapping to ensure that the explosion is big. The more pressure that build up
against the walls of the break, the bigger the explosion will be.
Casing: When the
fuse ignites the powder, there is a large volume of gas created in a short
period of time. The purpose of the casing in a firework is to contain that gas
until the pressure build up blasts the tube open.
Pyrotechnics vs. Fireworks:
The difference between pyrotechnics an fireworks is that pyrotechnics involve the calculation and making of fireworks. the end product of a pyrotechnics job are fireworks and the creation of a show. The technician has to calculate the exact angle and timing for the fireworks to go off. They deal with the safety aspect of the show as well. All the proper angles have to be calculated in order to ensure their safety and the safety of the audience. The technicians also set up the timing for when the show starts and ends. They are in control of the fuse box and need to know when an error occurred.
This page is not that of a real company, but is for a chemistry assignment for General Chemistry 140 at Monmouth College.