
SDS-PAGE Results
Gel electrophoresis is a method that separates macromolecules-either nucleic acids or proteins-on the basis of size, electric charge, and other physical properties. Gel electrophoresis refers to the technique in which molecules are forced across a span of gel, moved by an electrical current. Electrodes at either end of the gel provide the driving force. A molecule's properties determine how quickly an electric field can move the molecule through the medium. Two examples of this are agarose and polyacrylamide. Agarose gels have very large "pore" size and are used primarily to separate very large molecules with a molecular mass greater than 200 kdal. SDS-PAGE is another type of polyacrylamide electrophoresis. The pore size of the gel may be varied to produce different molecular separate effects for separating proteins of different sizes. Polyacrylamide gels offer greater flexibility and more sharply defined banding than agarose gels.
The gel used in this experiment was SDS-PAGE. SDS is the detergent used in the gel to break the non-covalent interactions between R-groups of the amino acids and mercaptoethanol will break covalent bonds. SDS is a detergent that binds tightly to protein and is negatively charged. Therefore, when it binds to all the proteins in a mixture, it gives them a charge that is roughly proportional to their molecular weight.
PAGE stands for PolyAcrylamide Gel Electrophoresis. There are two basic parts to each SDS-PAGE gel: a stacking gel and a resolving gel. The upper half of the gel is the stacking gel. This portion has a pH of 6.9 and has only 2-3% acrylamide, ensuring a "same start" for all samples loading into the gel. There are larger pores in this portion in order to concentrate a heavy band of all samples, before they travel to the stacking gel. The resolving gel is smaller-pored, making it easier for the molecules to be separated. The resolving gel is in the lower half of the gel; it is used to move the molecules through the gel. It has a pH of 8-9 and is greater than 7% acrylamide.
The samples and several standards and ladders are loaded into a gel and run vertically with some voltage running through them. The standards chosen were alcohol dehydrogenase, lysozyme, and bovine albumin. Two concentrations of each standard were made an then mixed with some loading dye. The samples and ladders also had dye added to them, making them visible to the naked eye. The sample were boiled then then run in the gel for approximately one hour with 135 volts running through them. After the gel was completed, the gel was put into Brillant Blue Stain to cause a shift in wavelength absorption to 595 nm, which is directly related to the concentration of the protein.
Here is a sample picture of what a SDS-PAGE gel looks like:

Information and picture found at: http://en.wikipedia.org/wiki/Image:SDS-PAGE.jpg
Here is a picture of the gel run in this experiment:
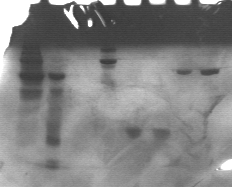
In the first two lanes, there are standards. These are used to determine the size of proteins in the gel. In lane one, there is a ladder with markers ranging from 199.6 kDa to 6.7 kDa. In lane two, a smaller ladder with ranges from 36.5 kDa to 3.9 kDa was used. To see exact weights of each band, click here. Each band in the ladder is a known molecular weight. The samples can be determined from these known weights.
Lanes three and four are two different concentrations of albumin (bovine). Lane three's sample cannot be seen on this gel. Lane four can and its band is visible at a much higher weight than what should be expected. It should be present at approximately 65 kDa, but it is found in the 160 kDa range. Although the exact reason this occurred cannot be determined, there may have been a problem with the concentrations. The dye shows that the protein did not move all together, leading to presumptions that there may be other proteins in there as well.
The next two lanes have lysozyme in them. The bands are found around 15 kDa. They are right next to one another, showing that there is only lysozyme found in those two lanes. The final two lanes have alcohol dehydrogenase, present at approximately 140 kDa. Again, they are almost directly next to one another. If GFP had been run on this gel, it would be found at 40 kDa. That is below the alcohol dehydrogenase and above the lysozyme, approximately in the middle of the gel.
From the gel above, it is determined that alcohol dehydrogenase and lysozyme are relatively pure proteins, meaning that there are few interferences with other proteins or molecules. It was also found that a protein will have the same molecular weight, regardless of the concentration.