How Lasers Work

Home Music Fireworks First Show
Atomic Structure of an Atom
To first understand how a laser works, you must first understand the structure of an atom.
An atom is a structure which consists of a nucleus, containing the protons and neutrons, and an electron could, containing the electrons. The electrons in the electron cloud circle the nucleus in a number of different orbits. Each different orbital represents a different energy level. The atom begins at a ground-state energy level, and if energy is applied to the atom, it leaves the ground-state level and goes to an excited level. The actual excited level that the atom reaches depends on how much energy is actually applied to the atom. Below is a picture of the simple structure of an atom.
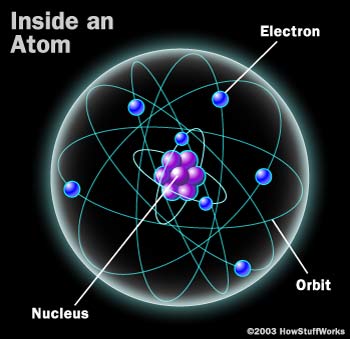
(Picture from: http://science.howstuffworks.com/laser1.htm)
Once an electron reaches an excited state, it will want to return to it's ground state. When it finally returns to it's ground state, a photon of energy is released. A photon is simply a small particle of light. Anything that produces light does so because of the process of electrons going from excited to ground states and releasing photons in the process.
Why Atoms are Important to Lasers
By definition, "a laser is a device that controls the way that energized atoms release photons." The word "laser" actually stands for "Light Amplification by Stimulated Emission of Radiation."
Even though there are many different types of lasers, they all have the same essential features. First off, "the lasing medium is 'pumped' to get the atoms into an excited state." This is typically done with concentrated flashes of light or electrical discharges. These light flashes or electrical discharges create a large collection of atoms at an excited state only about two or three levels higher than their ground state. This is important because it "increases the degree of population inversion," which by definition is "the number of atoms in the excited state versus the number in ground state."
Now the atoms contain some electrons that are in excited levels which basically means that they have more energy than electrons in their ground state. It takes energy to move the electrons up into an excited level, therefore, when the electron goes back down to it's ground state, it releases energy. This energy that is released is called a photon. A photon is light energy, and this photon gives off a certain "wavelength that depends on the state of the electron's energy when the photon is released." This wavelength is also where the color of the light comes from. When there are two atoms that are exactly the same with the electrons in the same states, these two atoms will release photons with the exact same wavelength.
The picture below illustrates how a photon is released.
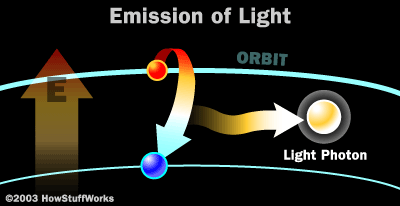
(Picture from: http://science.howstuffworks.com/laser3.htm)
Laser Light
Lasers have three basic properties that make them different from regular light.
The light emitted from a laser is monochromatic, which means that "it contains one specific wavelength of light (one color)." The wavelength, in turn, is determined by how much energy is released when the electrons drop into lower orbits.
Lasers also emit light that is "coherent" or "organized." This means that all the photons move together, and their "wave fronts launch in unison."
The light released by a laser is also very directional. A light from a laser goes in one specific direction and it is concentrated on that small area as oppose to a flashlight which emits light in one general area. The light from a flashlight is very weak compared to a laser light. See picture below.

(Picture from: http://www.technology.niagarac.on.ca/courses/tech238g/Lasers.html)
Stimulated emission occurs under specific circumstances. One way this can occur is if a particular photon, which has a specific wavelength, comes in contact with and "atom that has an electron in the same excited state." This particular photon can force a photon from a different atom to "vibrate with the same frequency and direction" as the first one.
That's only half the basics behind a laser. The other essential to a laser is a
set of mirrors. These mirrors are strategically placed with one on both sides of
the "lasing medium." The photons will reflect off the mirrors repeatedly
"through the lasing medium." This process, in turn, causes other electrons to
fall from their excited states and more photons are then emitted. The photons
that are emitted also have the "same wavelength and phase." One of the mirrors
around the "lasing medium" is "half-silvered". This means that it doesn't
reflect all of the light. It lets some go through, and this light is the "laser
light."
Different Types of Lasers
There are 5 different types of lasers, and many different kinds in each type.
Solid-state Lasers - Have a "solid matrix of lasing material."
Gas Lasers - Most common. Outputs a red light.
Excimer Lasers - "Use reactive gases mixed with inert gases." Can produce ultraviolet light.
Dye Lasers - Contain "complex organic dyes." These particular lasers are also "tunable over a broad range of wavelengths."
Semiconductor Lasers (Diode lasers) - Used in electronic devices. Are small and use little power.
Examples of Some Different Types of Lasers
1. Ruby Laser:
Contents:
* Flash Tube
* Ruby Rod
*Two Mirrors (one half-silvered)
How it works:
* The atoms in the ruby are excited by a flash of light emitted by the flash tube.
* Some photons are emitted from the atoms, and begin to bounce back and forth between the mirrors. As they are going back and forth, they force other atoms to release their photons as well.
* This results in "monochromatic, single-phase, columnated light" that exits through the half-silvered mirror, and that is how you get your laser light.
* Below is a drawing of how a ruby laser looks. Except the laser rod would be a ruby rod.
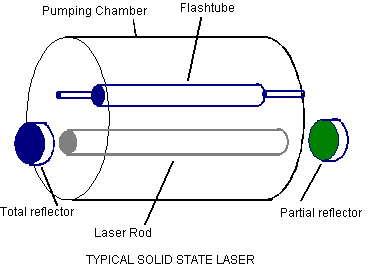
(Picture from: http://vcs.abdn.ac.uk/ENGINEERING/lasers/solidstate.html)
2. Neodymium: Yttrium-aluminum garnet laser (nd-YAG
This laser is exactly the same as the ruby laser but has a crystal of yttrium-aluminium-garnate instead of a rod of ruby.
3. Helium-Neon (HeNe) Laser:
Contents:
* Cathode and Anode
* Two Mirrors (one half-silvered)
* Gaseous mixture of He and Ne atoms
How it works:
* The gases are contained in a discharge tube.
* A discharge is created through electrical currents.
* The He atoms collide with electrons from the discharge, and, in turn, collide with the Ne atoms. The Ne atoms are then excited, and "population inversion builds up.
* Then photons are released and emit light in the way described earlier.
* Below are pictures of
how a HeNe laser is put together and what the light emitted looks like.


(Pictures from:
http://vcs.abdn.ac.uk/ENGINEERING/lasers/gas.html)
Resources:
Most of the resources used in this site were from www.howstuffworks.com, and also from http://vcs.abdn.ac.uk/ENGINEERING/lasers/lasers.html.
The picture used at the top is from:
http://science.howstuffworks.com/framed.htm?parent=laser.htm&url=http://laser.shows.org/.
This page is not that of a real company but is for a chemistry assignment for General Chemistry 140 at Monmouth College